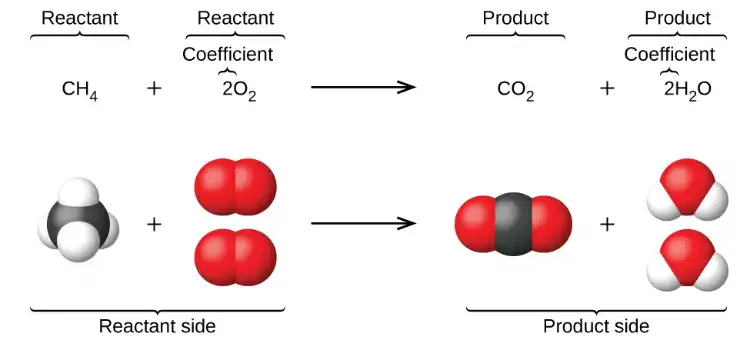
A balanced chemical equation is a chemical equation in which the number of atoms for each element in the reactants is equal to the number of atoms for that element in the products. This means that the equation is consistent with the law of conservation of mass, which states that the total mass of the reactants must be equal to the total mass of the products.
To balance a chemical equation, coefficients (numbers placed in front of the chemical formulas) are used to adjust the number of atoms of each element in the reactants and products. For example, the equation for the combustion of methane (CH4) is often written as: CH4 + O2 –> CO2 + H2O. However, this equation is not balanced because there is only one carbon atom on the reactant side, but two on the product side. To balance the equation, we can add a coefficient of 2 in front of the CH4: 2CH4 + O2 –> 2CO2 + 4H2O. Now, the equation is balanced and the law of conservation of mass is obeyed.
Balancing chemical equations is important for a number of reasons. Firstly, it ensures that the equation is consistent with the law of conservation of mass, which is a fundamental principle in chemistry. Secondly, balanced equations can be used to calculate the relative amounts of reactants and products needed to complete a reaction. This is important in chemical reactions as it allows us to determine how much of each reactant and product is required in order to produce a desired amount of product.
In addition, balanced chemical equations are necessary for calculating the amount of heat generated or absorbed in a reaction. The coefficients in the balanced equation can also be used to determine the number of moles of reactants and products involved in the reaction, which is important for understanding the stoichiometry of the reaction.
In conclusion, a balanced chemical equation is a chemical equation in which the number of atoms for each element in the reactants is equal to the number of atoms for that element in the products. Balancing chemical equations is crucial for understanding the stoichiometry of a reaction, determining the amount of heat generated or absorbed and also for calculating the amount of reactants and products required to complete a reaction.