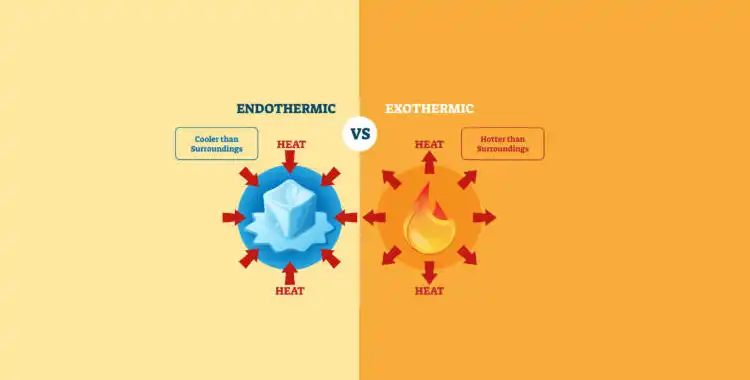
Exothermic and endothermic reactions are two types of chemical reactions that are characterized by the amount of heat that is either absorbed or released during the reaction.
An exothermic reaction is a chemical reaction that releases heat. Examples of exothermic reactions include combustion, such as the burning of wood or gasoline, and the rusting of iron. In these reactions, heat is given off as a byproduct, and the temperature of the surroundings increases.
On the other hand, an endothermic reaction is a chemical reaction that absorbs heat. Examples of endothermic reactions include photosynthesis, the process by which plants convert light energy into chemical energy, and the dissolution of ammonium nitrate in water. In these reactions, heat is absorbed, and the temperature of the surroundings decreases.
Overall, the difference between exothermic and endothermic reactions is the direction of heat flow. In exothermic reactions, heat flows from the system to the surroundings, while in endothermic reactions, heat flows from the surroundings to the system. Understanding the difference between these two types of reactions is important in fields such as chemistry, thermodynamics, and biochemistry.
In summary, exothermic reactions release heat and increase the temperature of the surroundings while endothermic reactions absorb heat and decrease the temperature of the surroundings. Understanding the difference between these two types of reactions is important in fields such as chemistry, thermodynamics and biochemistry.
What is common or similarity between exothermic and endothermic reactions?
Both exothermic and endothermic reactions involve a change in the amount of heat energy within the system. In exothermic reactions, heat is released and the temperature of the surroundings increases, while in endothermic reactions, heat is absorbed and the temperature of the surroundings decreases.
Another common feature between exothermic and endothermic reactions is that both types of reactions involve a change in the chemical bonds between atoms or molecules. In exothermic reactions, chemical bonds are broken and new bonds are formed, releasing heat energy in the process. In endothermic reactions, new bonds are formed and energy is absorbed from the surroundings.
Additionally, Both exothermic and endothermic reactions are thermodynamically controlled meaning that the heat energy change is determined by the difference in the free energy of the reactants and products.